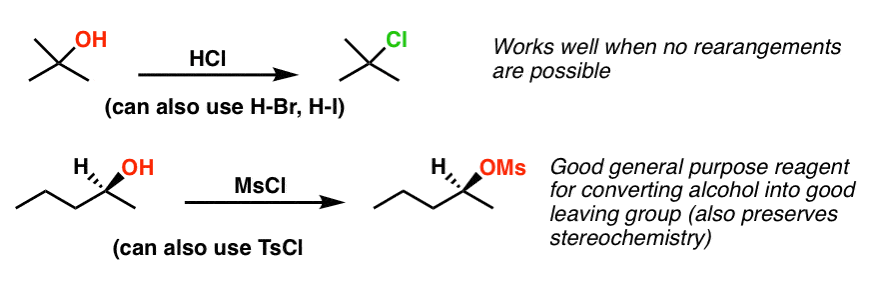
Reaction type Nucleophilic Substitution (S N 1 or S N 2) Summary Alcohols can also be converted to alkyl chlorides using thionyl chloride, SOCl 2, or phosphorous trichloride, PCl 3;CH3OH and most 1o alcohols react with HX via SN2 mechanism 3o and 2o react with HX via SN1 mechanism Both mechanisms include an additional, first step, protonation of the alcohol oxygen ROH H ROH2 "oxonium ion" Whenever an oxygen containing compound is placed into an acidic solution, the oxygen will be protonated, forming an oxoniumCreate Phosphorus oxychloride appears as a colorless fuming liquid with a pungent odor Density 140 lb / gal Very toxic by inhalation and corrosive to metals and tissue Used in gasoline additives and hydraulic fluids CAMEO Chemicals Phosphoryl trichloride is a phosphorus coordination entity
2
R o p classes
R o p classes-The –OH is a poor leaving group, but –OH 2 is an excellent leaving group, and once the OH is protonated, the molecule may take part in a variety of substitution and/or elimination reactions The nature of R determines whether the reactions proceed via S N 1 or S N 2 mechanisms If R is primary alkyl S N 2 If R is bulky tertiary alkyl S N 1 Solution Alkyl alcohol is mixed with phosphorous trichloride and alkyl chloride is formed along with Pyridine in the above reaction 14k 278k Complete the following (i) (ii)
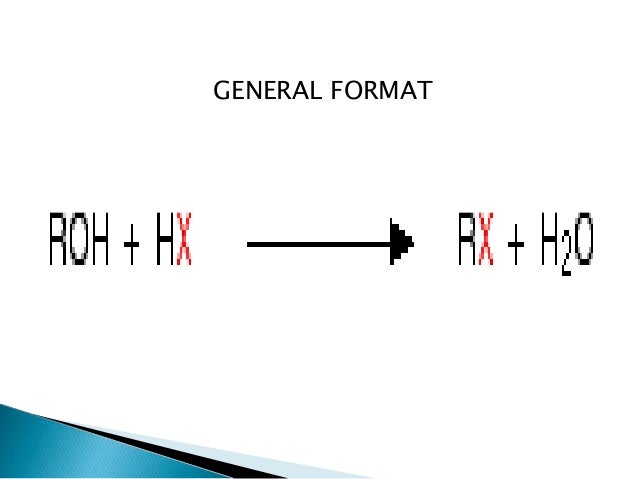



Conversion Of Alcohols To Halide
R'OH cat H2SO4 SOCl2 PCl3 (COCl)2 PBr3 P2O5, heating H2NR DCC H2O, H heating Title handoutacid03PDF Author chang Created Date 2/24/03 AMClick here👆to get an answer to your question ️ Which of the following is the byproduct when R OH is reacted with PCl3 ?Instructions To balance a chemical equation, enter an equation of a chemical reaction and press the BalancePKa Data Compiled by R Williams ACIDS Compound pK Ref H3PO2 , 223* 28 H2PO4– 721* 77 AgOH 396 4 HPO4_ 1232* 77 Al(OH)3 112 28 As(OH) H3PO3 28 3 922 28 H3AsO4 222, 70, 130 28 H2PO3– 658* 77Answer (1 of 3) 2512 Hofmann Elimination 1 picture_as_pdfLMSDonate Amine functions seldom serve as leaving groups in nucleophilic substitution or basecatalyzed elimination reactions Indeed, they are even less effective in this role than are hydroxyl and alkoxyl groups In the case of alcoh
2R – OH → R – O – R H 2 O (H 2 SO 4, 140 o C) Contoh 2CH 3 – OH → CH 3 – O – CH 3 H 2 O (H 2 SO 4, 140 o C) Eter tidak dapat bereaksi dengan PCl3, tetapi dapat bereaksi dengan PCl5 karena mudah mendapatkan energi Namun, dalam reaksi alkohol PCl5 pasti menghasilkan HCl, tetapi pada eter tidak menghasilkan HClAcetyl chloride (CH 3 COCl) reacts with grignard reagent and produce tertiary alcohol when H 2 O is added in the final stage If we denote grignard reagent as, RMgBr Product will be CH 3 C(R) 2 OH Related Tutorials to CH 3 MgBr and CH 3 COClMy New CHANNEL (A square Vlogs)LINK Click And Subscribe Now https//wwwyoutubecom/channel/UC6ERimtc5zFrn7x6Bk3HaHAemail id madeejeeyt@gmailcomMY INSTAGR
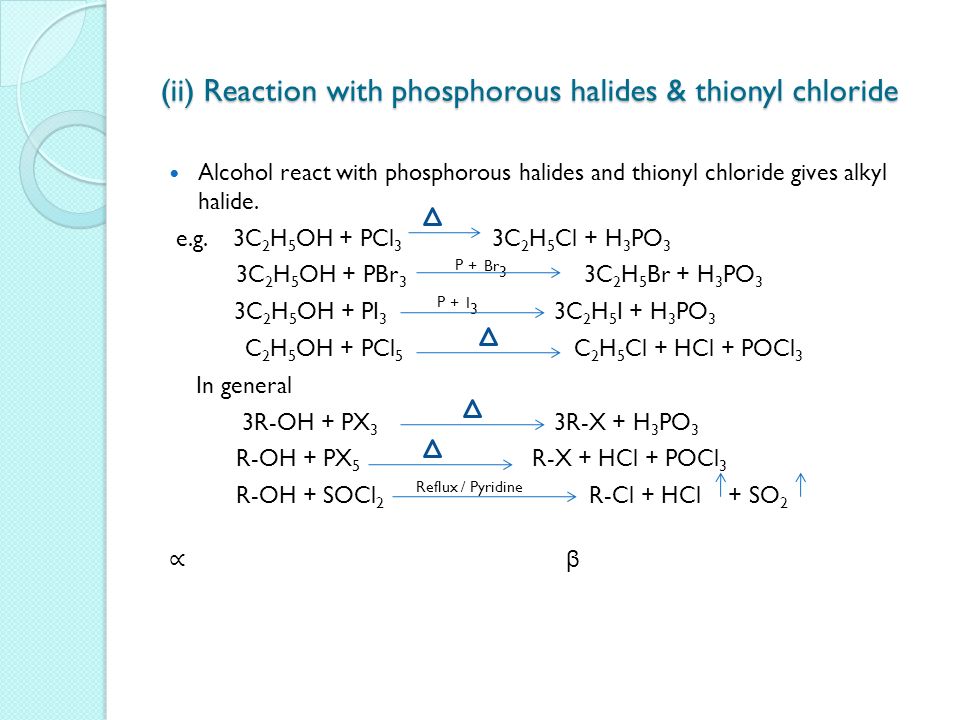
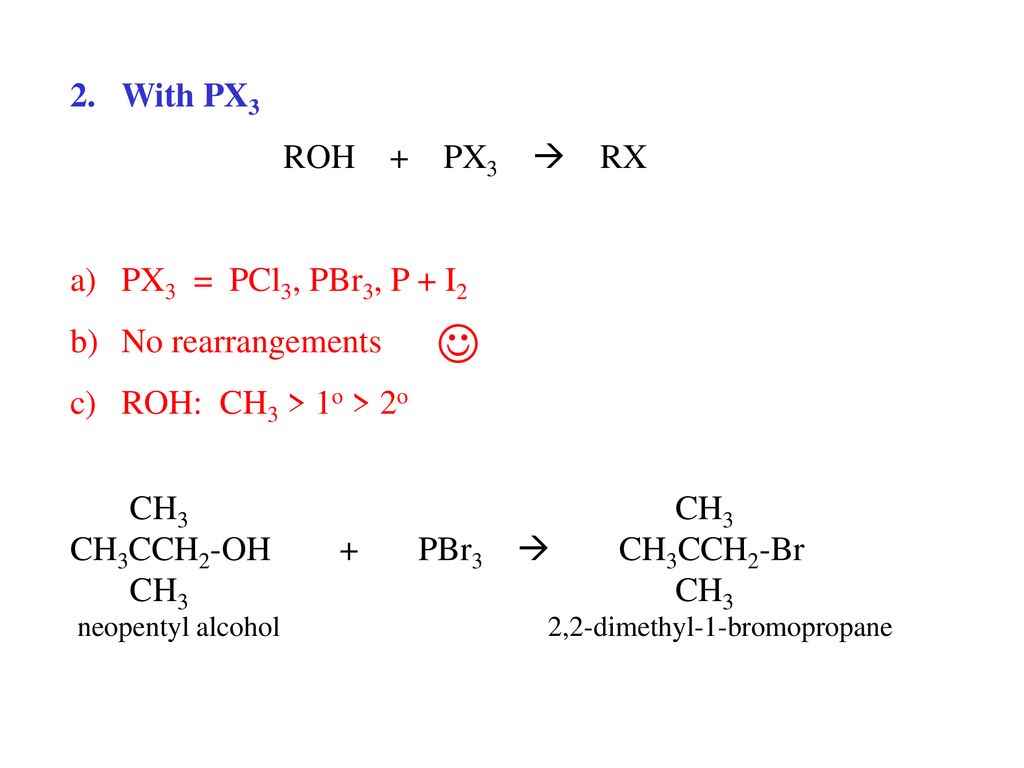
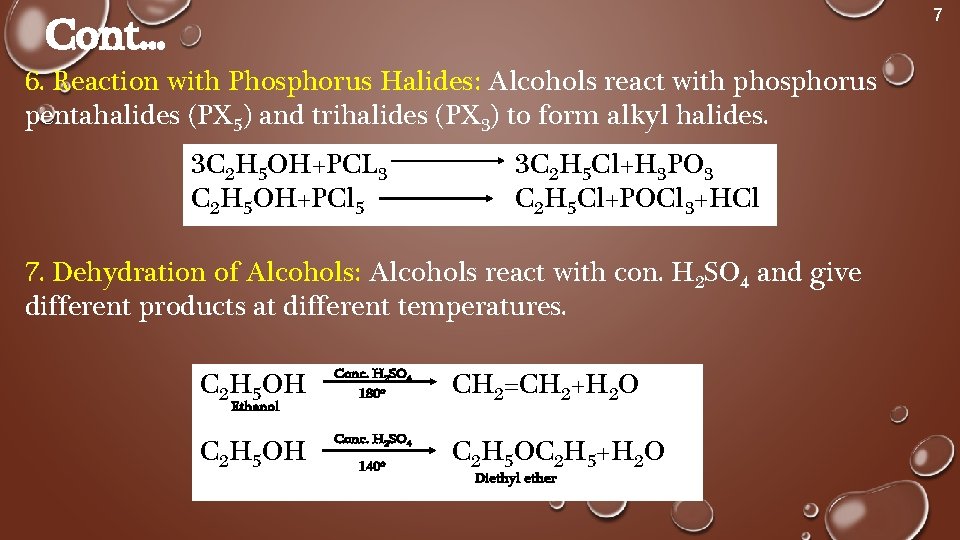
3 ROH PCl 3 3 RCl H 3 PO 3 Alcohol phosphorous trihalide alkyl halide phosphorous acid Example – 1 Preparation of ethyl chloride (Chloroethane) from ethyl alcohol (Ethanol) 3C 2 H 5 OH PCl 3 3C 2 H 5 Cl H 3 PO 3RCOOH R'OH (H)> RCOOR' PCl3, R'OH RCOOHPCl3> RCOClR'OHBase(Et3N,pyr,)>RCOOR' HCl t OH RCOCl tOH> RCOtbulky SO2Ar forces Nu to react at carbonyl and sulfonate will leaveCan also use TsCl, TsBr Other sets by this creator EEMB 2 Evolution 11 terms3r−ohpcl3 3r−clh3po33r−ohpclx3 3r−clhx3pox3 R−OHSOCl2 R−ClSO2↑HCl↑R−OHSOClX2 R−ClSOX2↑HCl↑ Both the byproducts formed in the last reaction ie,SO2SOX2andHClHClare gases and can easily escape out into the atmosphere, so the equilibrium shifts more to the right side and hence, more products are obtained




L 15 R Cooh Reaction With Socl2 Pcl5 Pcl3 With Mechanism Jee Neet Youtube



2
Used mostly for 1 o and 2 o ROH (via S N 2 mechanism); What is the mechanism of 3(ROH PCl3) Chemistry Haloalkanes and Haloarenes NCERT Solutions;In a Hoechst continuous process, molten white phosphorus and gaseous chlorine react in previously produced phosphorus trichloride The formation of phosphorus pentachloride is prevented by the presence of a small excess of phosphorusThe heat of reaction, ca 10 times the heat of evaporation, keeps the system at its boiling point, and the phosphorus trichloride distills off
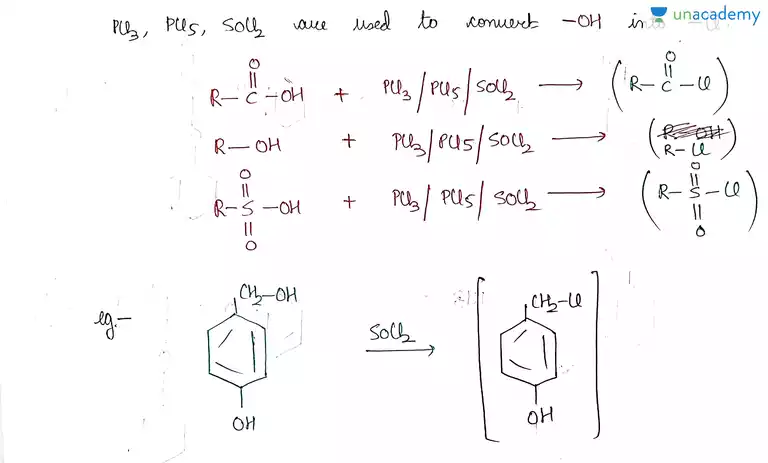



Iit Jee Reaction With Pcl5 Pcl3 Socl2 And Hx Offered By Unacademy
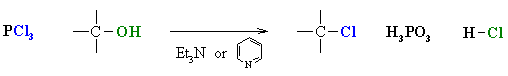



Ch 8 Roh Socl2 Or Px3
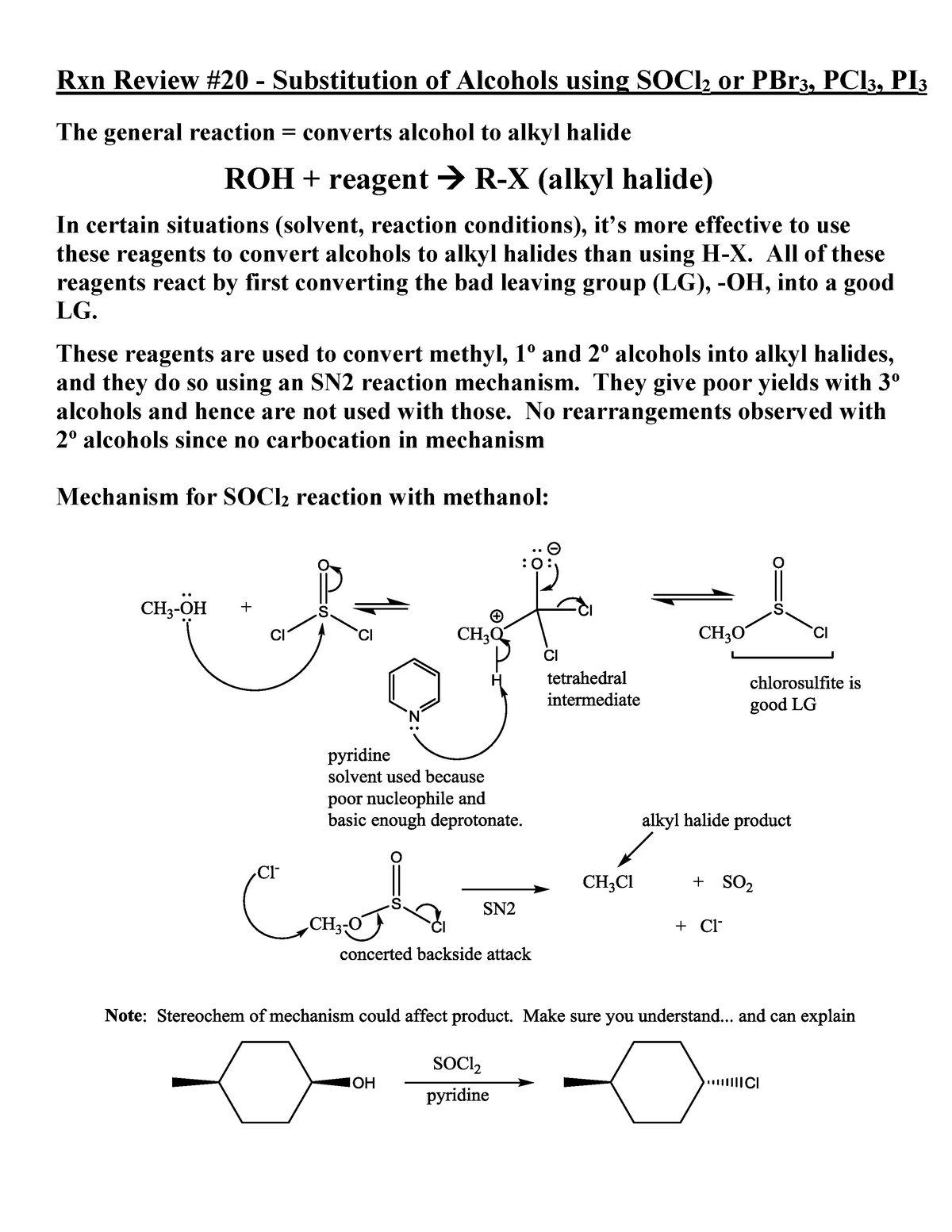
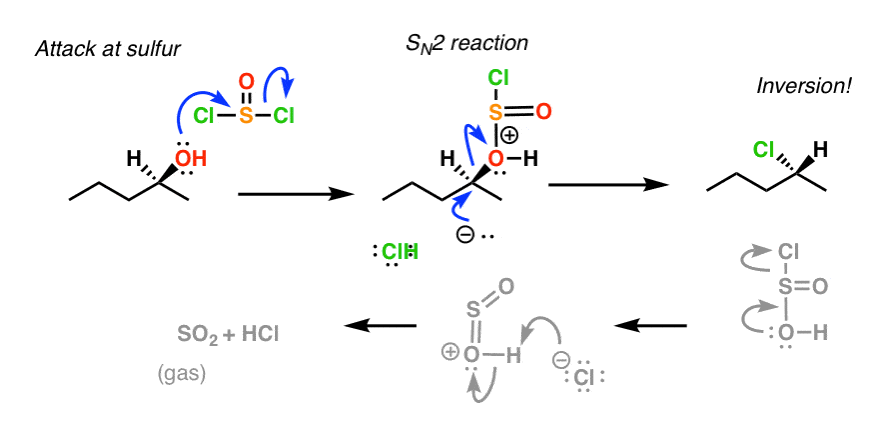
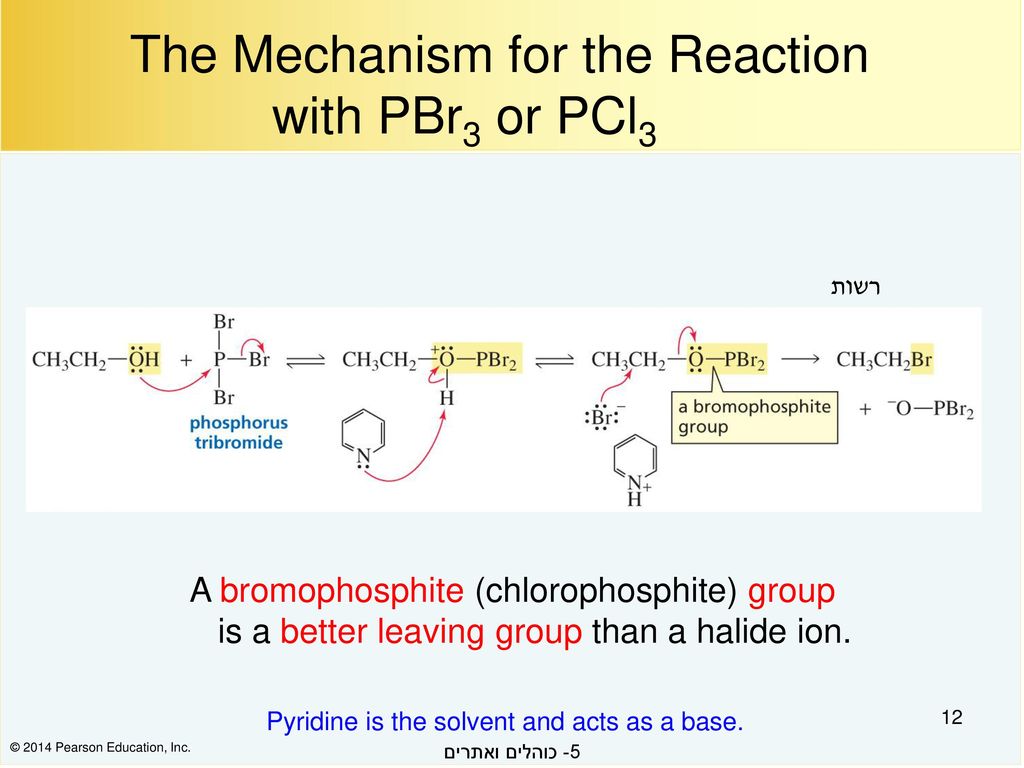
Both reactions have similar mechanisms with the idea of turning the OH into a good leaving group and then replacing it with the Cl – or Br – nucleophile via an S N 2 reaction Let's start with SOCl 2 Reaction of Alcohols with SOCl 2 The alcohol reacts with SOCl 2 to form an intermediate that is deprotonated by pyridine in the next step These two reactions convert the OH group into a (1) Alcohols containing C sp 3 – OH bond In these alcohols OH group is attached to a sp 3 – hybridised carbon atom of alkyl group These alcohols are represented as ROH They are further classified as primary, secondary and tertiary alcohols in which – OH group is attached to primary, secondary and tertiary carbon atoms respectively3r−ohpcl3 3r−clh3po33r−ohpclx3 3r−clhx3pox3 R−OHSOCl2 R−ClSO2↑HCl↑R−OHSOClX2 R−ClSOX2↑HCl↑ Both the byproducts formed in the last reaction ie,SO2SOX2andHClHClare gases and can easily escape out into the atmosphere, so the equilibrium shifts more to the right side and hence, more products are obtained




Phosphorus Trichloride An Overview Sciencedirect Topics




Phosphorus Trichloride An Overview Sciencedirect Topics
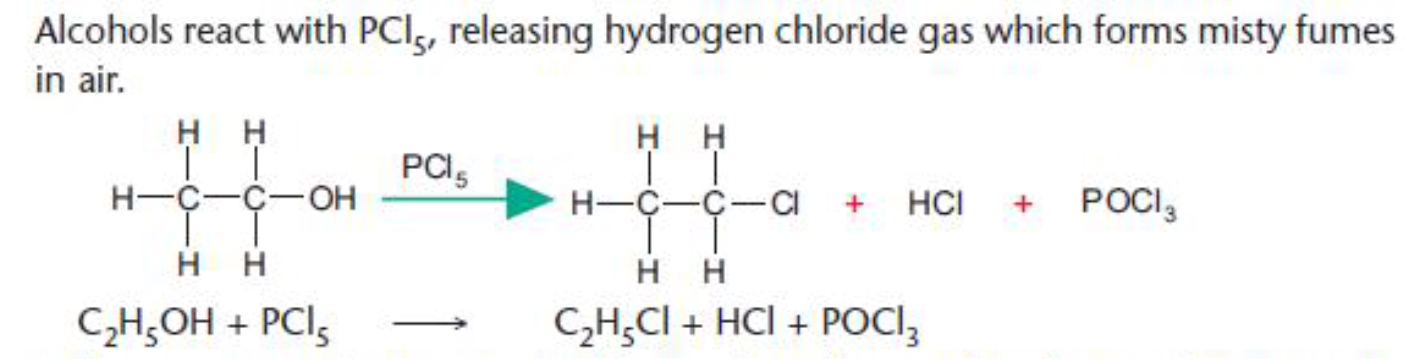
H 3 P O 3 is the byproduct when RCl is reacted with P C l 3 3 R − O H P C l 3 → R − C l H 3 P O 3 In the final product, we get a byproduct of H 3 P O 3ROH PCl5 → RCl POCl3 HCl C2H5OH PCl5 → C2H5– Cl POCl3 HCl एल्कोहल (alcohol) की क्रिया PCl3 से करने पर 3(C2H5OH) PCl3 → H3PO3 3C2H5Cl Re Reagents and conditions for reactions in organic chemist alcohol> chloroalkanes 1 Use PCL5, By products POCL3, HCL 2 PCL3, By products H3PO3 3 SOCL2 By products S02 HCL alcohols> Bromoalkanes 1 Used red phosphorous 4PBR2 2 Use HBR gas, which is made by the following
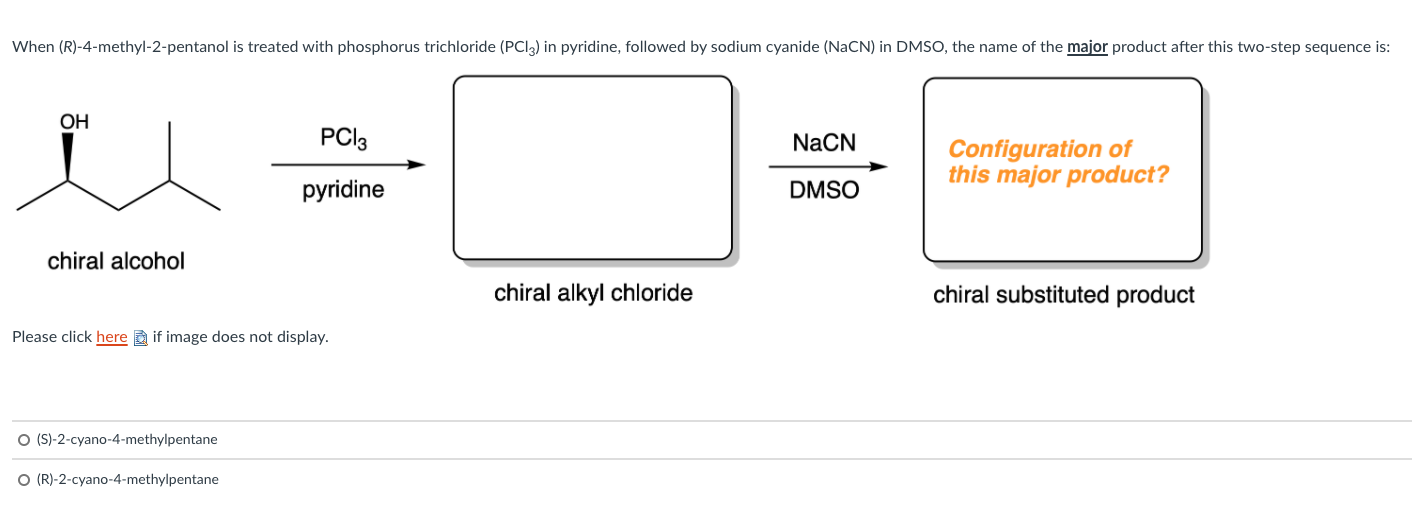



Solved When R 4 Methyl 2 Pentanol Is Treated With Chegg Com
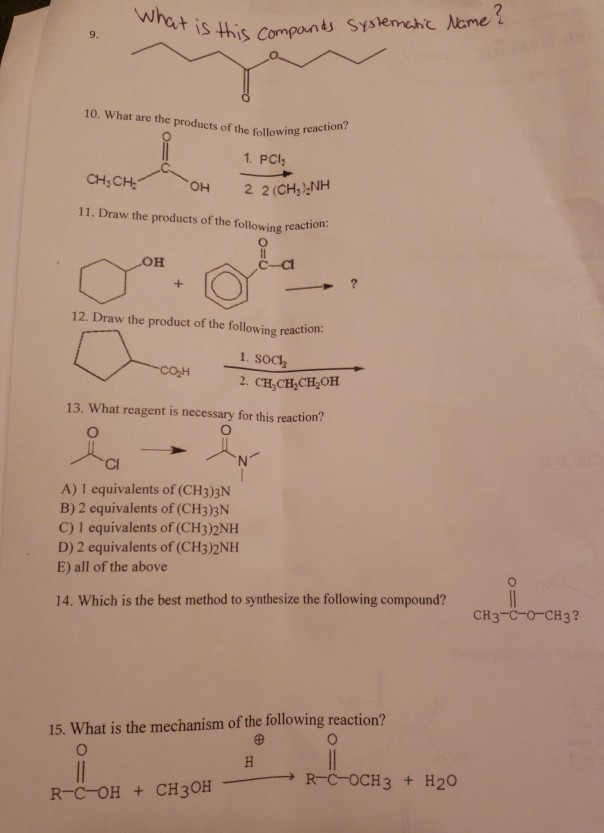



Solved 9 10 What Are The Products Of The Following Rea 1 Chegg Com
R – OH PCl5 → R – Cl PCl3 HCl Contoh CH3CH2OH PCl5 → CH3CH2 – Cl PCl3 HCl Pembebasan hidrogen klorida (HCl) dari reaksi alkohol dan PCl5 adala sifat khas yang biasanya digunakan untuk identifikasi ada atau tidaknya gugus OH dalam suatu senyawaComplete the following reaction ROH PCl5 →\rightarrow→ RCl X HCl X is PCl3, PCl5, POCl3, POBr3Alkenes from alcohols POCl3 Alkenes from alcohols POCl3 Definition When treated with phosphorus oxychloride (POCl3) alcohols are converted to alkenes via an elimination reaction Alkenes from alcohols POCl3 Explained As we know, hydroxide (OH‾) is a very poor leaving group In order for alcohols to participate in substitution and elimination reactions, it is the best




Which Of The Following Is Produced When R Oh React With Pcl3




Consider The Following Reaction R Oh Px5 R X B Hx What Is B
Pada suhu dan volume tetap, 1 mol PCl5 (g) terurai menjadi PCl3 (g) dan Cl2 (g) Jika tetapan kesetimbangan (Kp) adalah 4/15 dan setelah kesetimbangan tercapai tekanan total menjadi 1,4 atm, maka derajat disosiasi PCl3 adalah A % B 30% C 40% D 50% R – OH PCl5 → R – Cl PCl3 HCl Contoh CH3CH2OH PCl5 → CH3CH2 – Cl PCl3 HCl Pembebasan hidrogen klorida (HCl) dari reaksi alkohol dan PCl5 adala sifat khas yang biasanya digunakan untuk identifikasi ada atau tidaknya gugus OH dalam suatu senyawaROH > R OH alcohol carbocation Hydroxide ion Mixed reaction Involving alkyl and OH group Reaction as nucleophiles) between C and O, RC*OH 1 Reaction with PCl5, PCl3, SOCl2 and HX 2 Lucas test and its observations (turns cloudy) Reaction of 1°, 2°, 3° ROH with ZnCl2 conc HCl 3 Dehydration reaction (2 types) Reaction with hot concentrated sulphuric acids a primary alcohol (1° ROH) with one alpha hydrogen



1
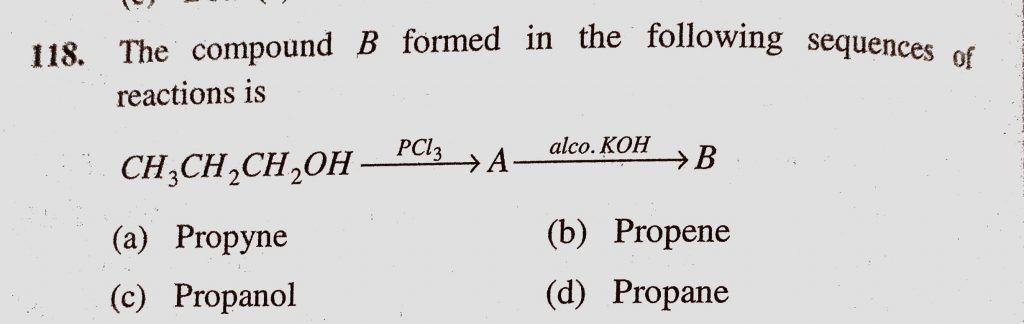



The Compound B Formed In The Following Sequence Of Reactions Is Ch3ch2ch2oh Pcl3 A Alco Koh To B A Propyne B Propene C Propanol D Propane Sahay Lms
Hydrogen chloride ROHPCl3=RClH3PO3HCl Wiki User ∙ This answer isCH 3 COOH PCl 3 dry ether → CH 3 COCl;Phosphorus trichloride is a inorganic compound with the chemical formula PCl 3 A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds It is toxic and reacts violently with water to
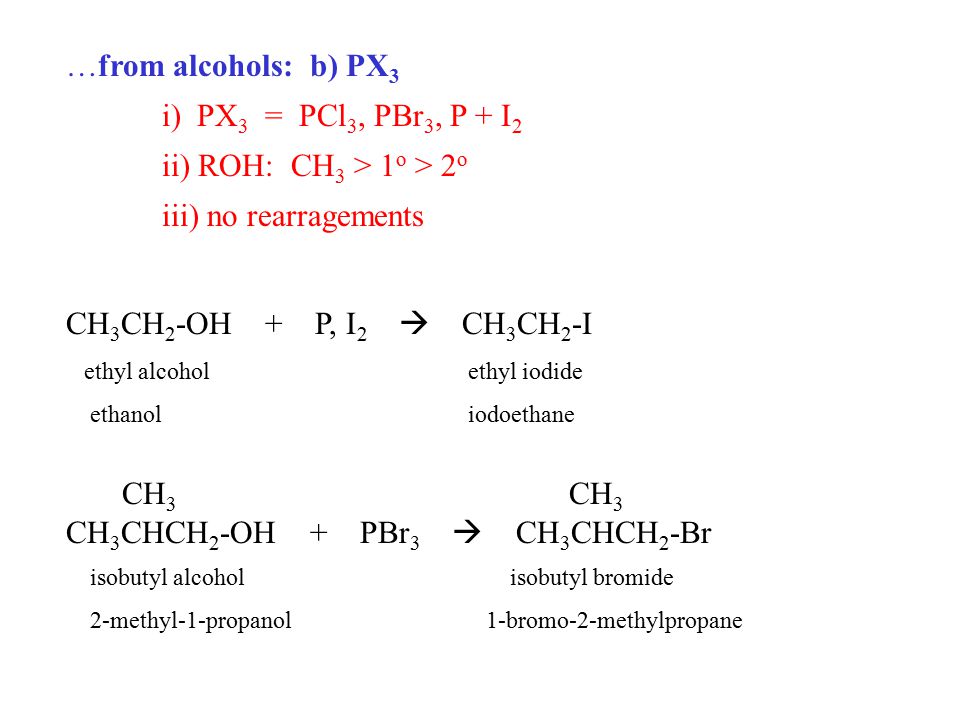



Alkyl Halides R X X F Cl Br I Ppt Video Online Download
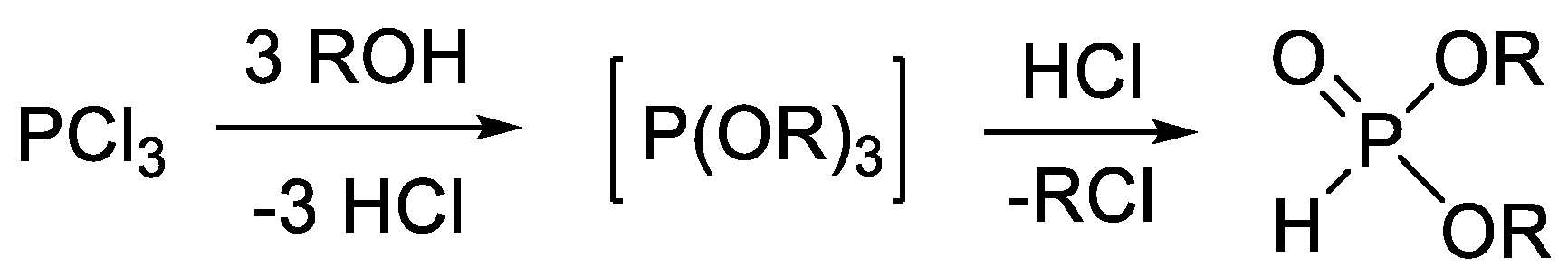



Molecules Free Full Text Continuous Flow Alcoholysis Of Dialkyl H Phosphonates With Aliphatic Alcohols Html
Need Help In Organic Chemistry I want a free source for studying organic chemistry upto jee mains level and they should be short and concise We are currently experimenting with this megathread thingy so please as to reduce text posts on the sub and create one place to seek advice, shitpost and vent out So please join JEE/NEET lounge 21Phosphorus trichloride (PCl3) is used as a chemical intermediate to produce a PSSPhosphorusTrichloridepdf Read/Download File Report lead to rearranged products Phosphorous Halides Phosphorous halides can convert alcohols to alkyl halides Eg 3 ROH PCl3 Ch11pdf Read/Download File Report Abuse phosphorous chemicalsPOCl3 for Dehydration of Alcohols Dehydration of alcohols using phosphorus oxychloride (POCl 3) and pyridine (an amine base) in place of H 2 SO 4 or TsOH is a good alternative for converting alcohols to alkenes when working with compounds that decompose in the presence of strong acids Let's compare the mechanisms of acidcatalyzed
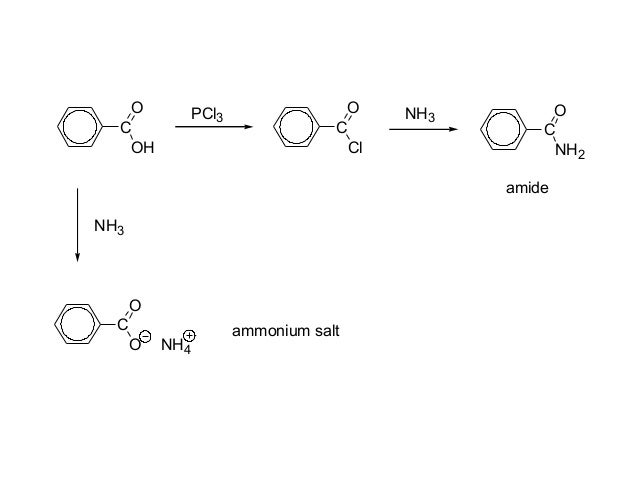



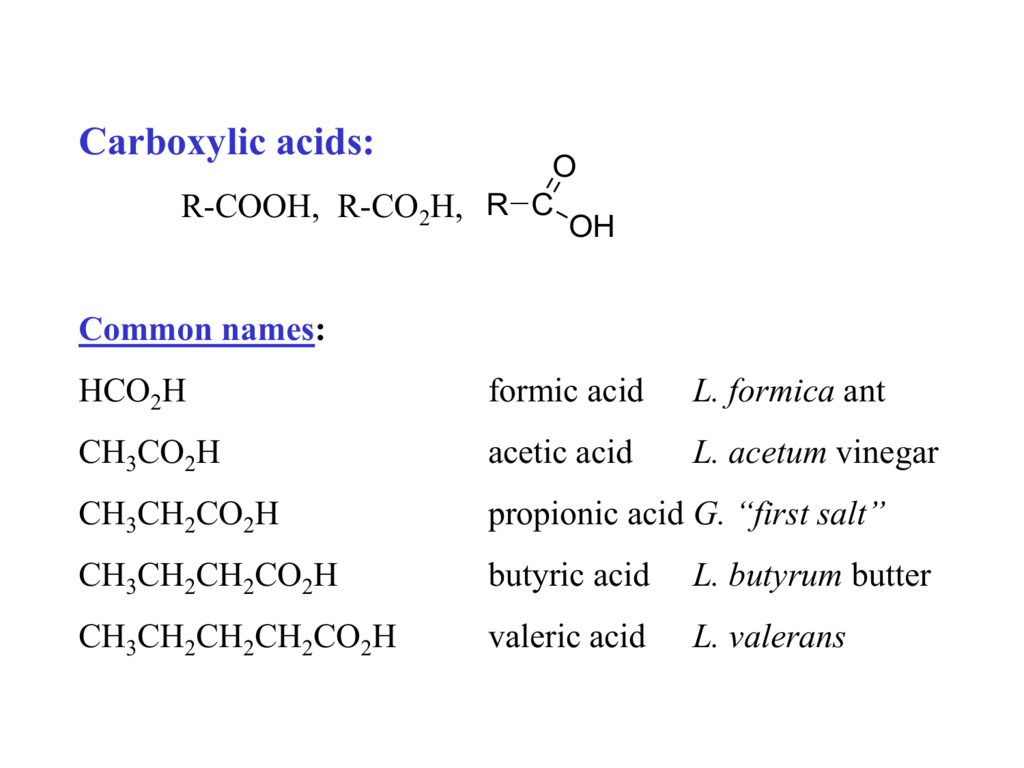
Carboxylic Acids
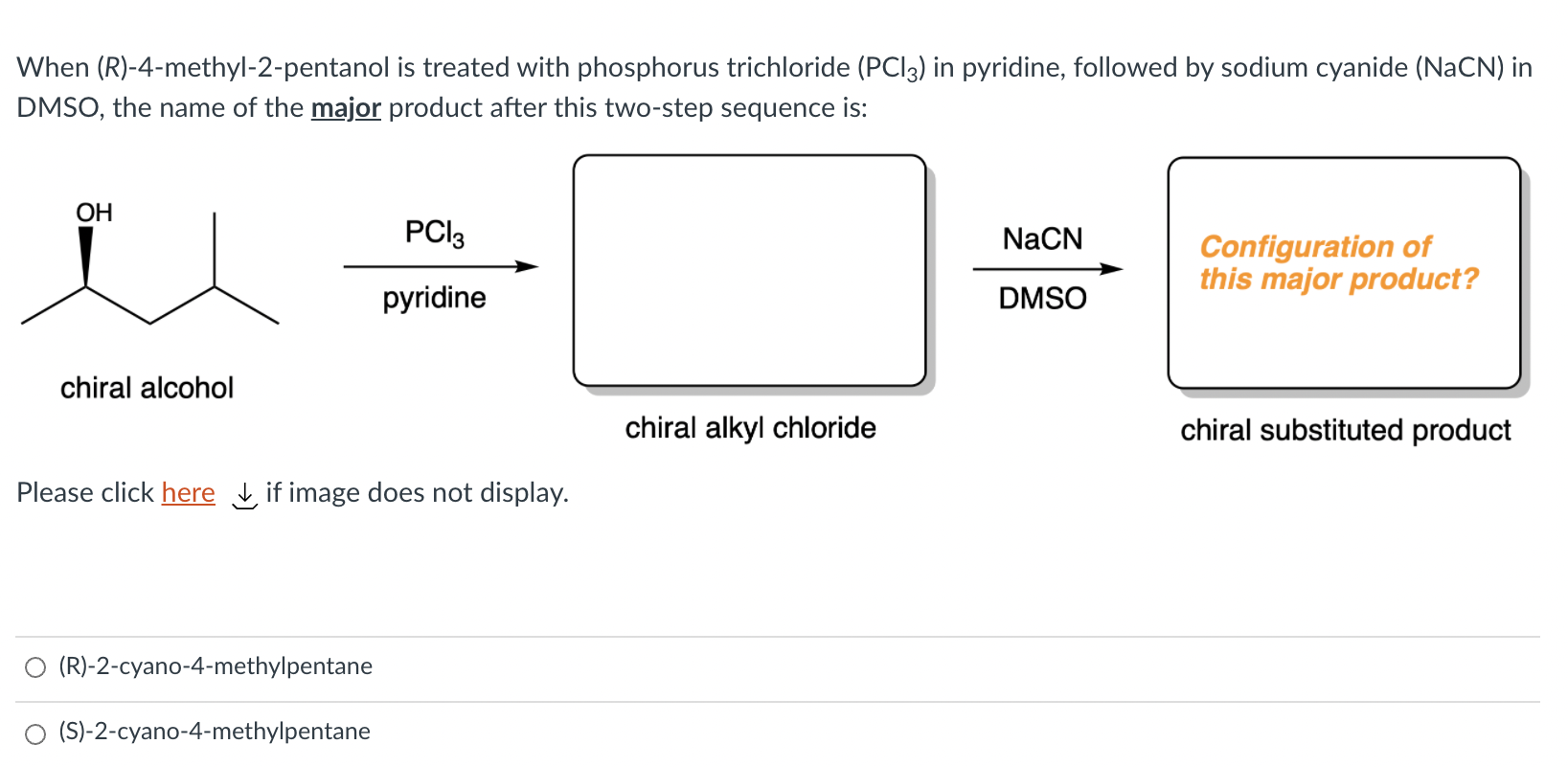



Solved When R 4 Methyl 2 Pentanol Is Treated With Chegg Com
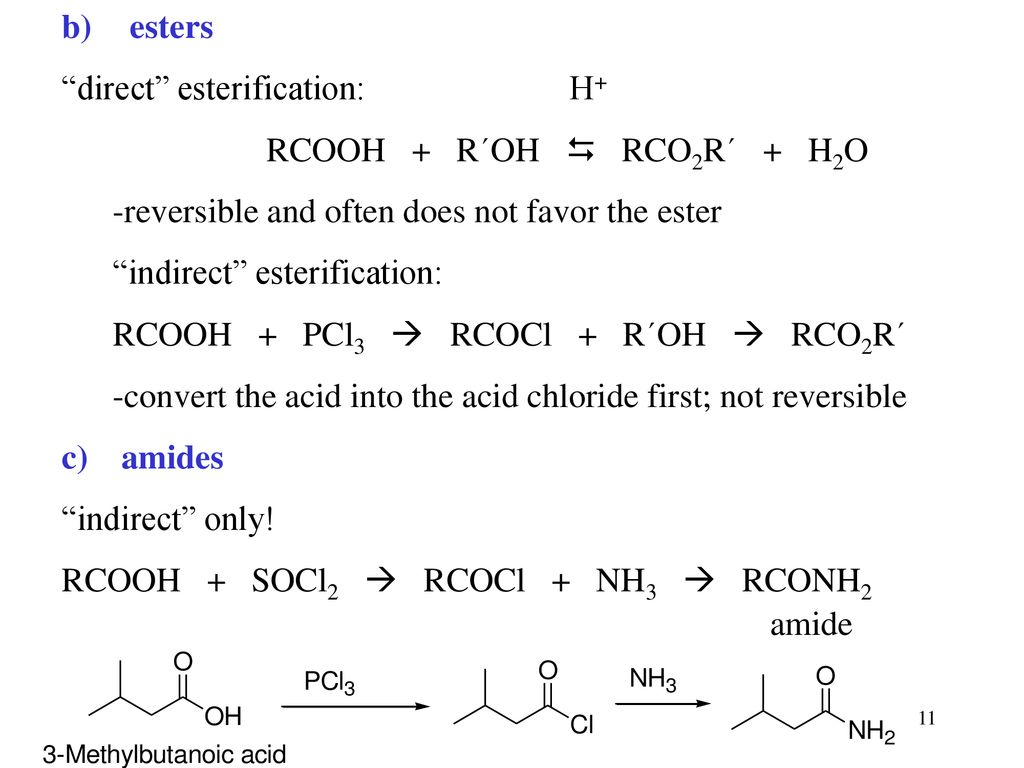
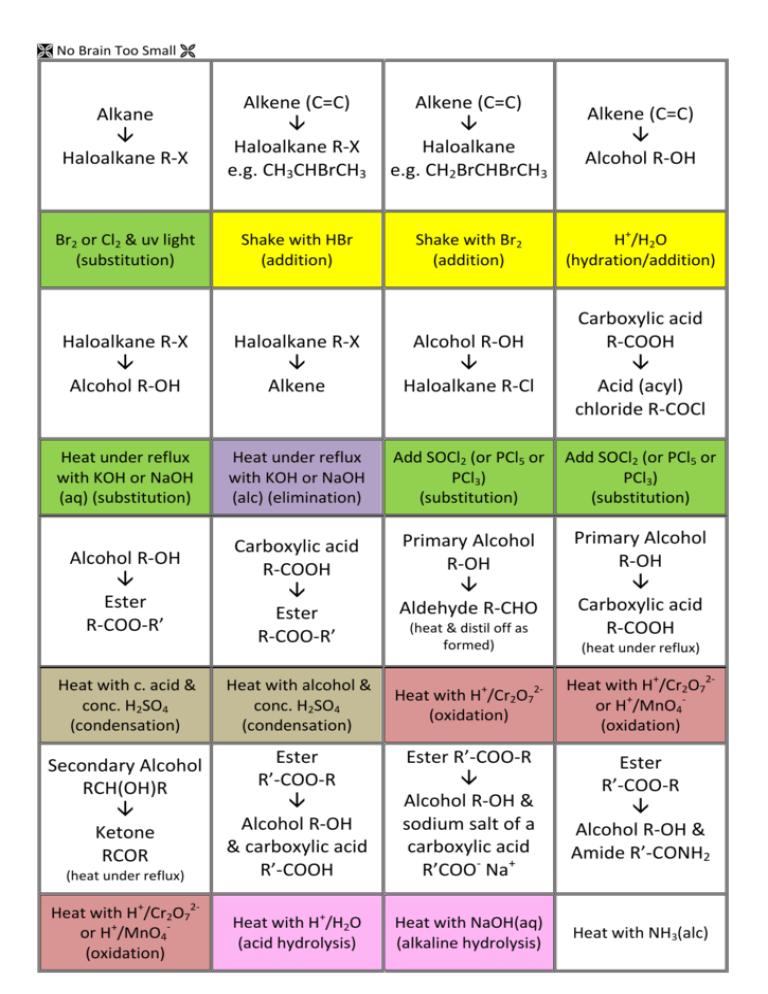
Conversion into functional derivatives a ) acid chlorides O R C OH SOCl2 O R C Cl or PCl3 orPCl5 CO2H SOCl2 CH3CH2CH2 C O OH COCl PCl3 CH3CH2CH2 C O Cl 30 b ) esters "direct" esterification H RCOOH R´OH RCO2R´ H2O reversible and often does not favor the ester use an excess of the alcohol or acid to shift equilibrium or removeAlkyl bromides can be prepared in a similar reaction using PBr 3;Reaction of carboxylic acid with alcohol, pcl3,pcl5,socl2,NH3
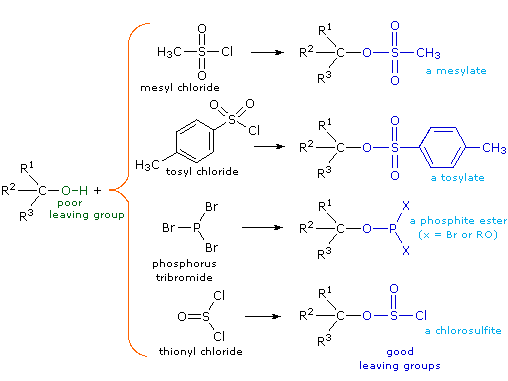



Alcohol Reactivity




Draw The Curved Arrow Mechanism For The Reaction Between 2s 3s 3 5 Dimethylhexan 2 Ol And Pcl3 Brainly Com
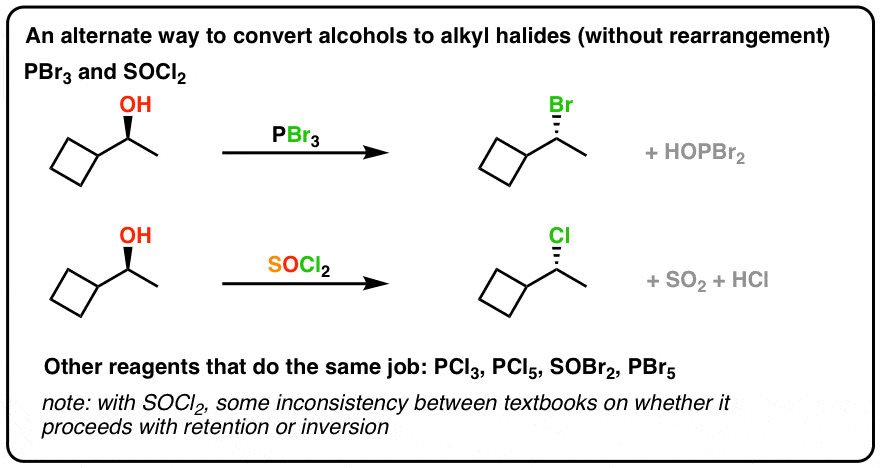
Answer (1 of 3) There are many processes through which we can prepare alkyl halides such as 1 From alcohols ROH SOCl2 → RCl SO2 HCl ROH Pcl3 → Rcl POcl3 HCl ROH PBr3 → RBr ROH Red P I2 → RI ROH dry conc HCl anh ZnCl2 → RRO R amine RN H 2 RS R R N R R R R quaternary ammonium ion pKa of conjugate acid of the leaving group −10 to 32 ∼15 ∼15 40 ∼10 sulfonate ester RO S O O R ∼ −6 ∼ −6 101 NucLeophiLic SuBStitutioN ReactioNS of aLcohoLS foRmiNG aLkyL haLiDeS An alcohol has a strongly basic leaving group (HO) that cannot be displaced by a $\begingroup$ Oh sorry my mistake, I of course meant $\ce{HPCl3}$ But to cut things a little shorter here HCl is a strong acid, and chloride is a terrible base This molecule will be (at least in most protic solvents) dissociated



1



What Is The Mechanism For A Reaction Between Alcohol And Phosphorus Pentachloride Quora
The carboxylic acid chemical structure contains a carbonyl functional group and hydroxyl group Anhydrides react rapidly to form esters, amides, N‐substituted amides, and carboxylic acids It interact easily with polar compounds and contributes to many important chemical reactions Replacement of OH replacement of OH by X using PX3 or (for Cl) SOCl2 eg Preparation of acid Oxidation H 1 alcohol R C OH Pyridinium chlorochromate (PCC) H CH2Cl2, 25oC RC=O H 1o alcohol aldehyde H R C OH Cu or Cr3O/pyridine H RC=O H o 1 alcohol Cr3O/pyridine = Collins reagent aldehyde H R C OH H o 1 alcohol KMnO4/H or K2Cr2O7/H or CrO3/H O RCOH carboxylic acid 54CH 3 CH 2 OH H KMnO 4 → CH 3 COOH;
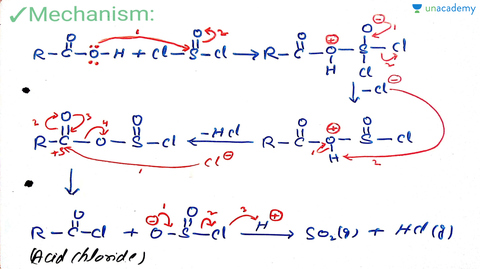



Neet Ug Reaction With Pcl5 Pcl3 And Socl2 And Reaction With Ammonia Offered By Unacademy



Org Chem Text Chapter 12 12 8 Htm
3 R — OH PCl3 ¾¾¾® 3R — Cl H3PO3 Alcohol alkyl chloride phosphorous acid 3 C2H3OH PCl3 ¾¾¾® 3 C2H5 — Cl H3PO3 Ethyl alcohol ethyl chloride (ii) Action of thionyl chloride (SOCl2) Alcohols react with thionyl chloride in the presence of pyridine (a base) to form the corresponding alkyl chloride, SO2 gas and HCl gasThis problem has been solved! What gas is produced when an alcohol reacts with pcl3?




In The Following Reaction Identify The Compound A 3ch3 Ch2 Oh Pcl3 3c2h5 Cl A



Alcohols Phenols Ethers Ppt Download
Complete the following (i) `ROH PCl_(5) rarr` (ii) `ROHPCl_(3) rarr` Books Physics NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless Chemistry NCERT P Bahadur IITJEE Previous Year Narendra Awasthi MS Chauhan Biology NCERT NCERT Exemplar NCERT Fingertips Errorless Vol1 Errorless Vol2See the answer Select only the oxygen atom of the reactive alcohol Select each alcohol functional group present in the following molecule that when treated with PCl3 or PBr3 would yield an alkyl halide at its positionRoh pcl3 Roh pcl3CH3 CH2 OH → ^PCl3 P → Δ ^alcKOH Q 'Q' is 12th Chemistry Alcohols, Phenols and Ethers Chemical Reactions of Alcohols and Phenols CH3 CH2 OH → ^PCl3 PCompute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals For math, science, nutrition, history



Reaction Review Substitution Of Alcohols Using Socl2 Pcl3 Pbr3 Studocu



Pbr3 And Socl2 Master Organic Chemistry
1 Replacement of OH replacement of OH by X using PX3 or (for Cl) SOCl2 eg RC O OH PCl3 heat RC O Cl P(OH)3 acyl chloride very reactive 2 Reduction of C=O to CH2 with LiAlH4 to give a primary alcohol RC O OH LiAlH4 ether RCH2OM M is Al or Li H3O RCH2OH eg H3CCH CH3 CH2 COOH 1) LiAlH4 2) H 3O H3CCH CH CH2 CH2OH 90 99% yield 3In each case a base is used to "mopup" the acidic byproduct $$\ce{3ROH PCl3 → 3RCl H3PO3}$$ $$\ce{ROH SOCl2 → RCl SO2 ↑ HCl ↑}$$ Both the byproducts formed in the last reaction ie, $\ce{SO2}$ and $\ce{HCl}$ are gases and can easily escape out into the atmosphere, so the equilibrium shifts more to the right side and hence, more products are obtained




Conversion Of Alcohols To Halide



Pbr3 And Socl2 Master Organic Chemistry




Socl2 And Pbr3 Chemistry Steps
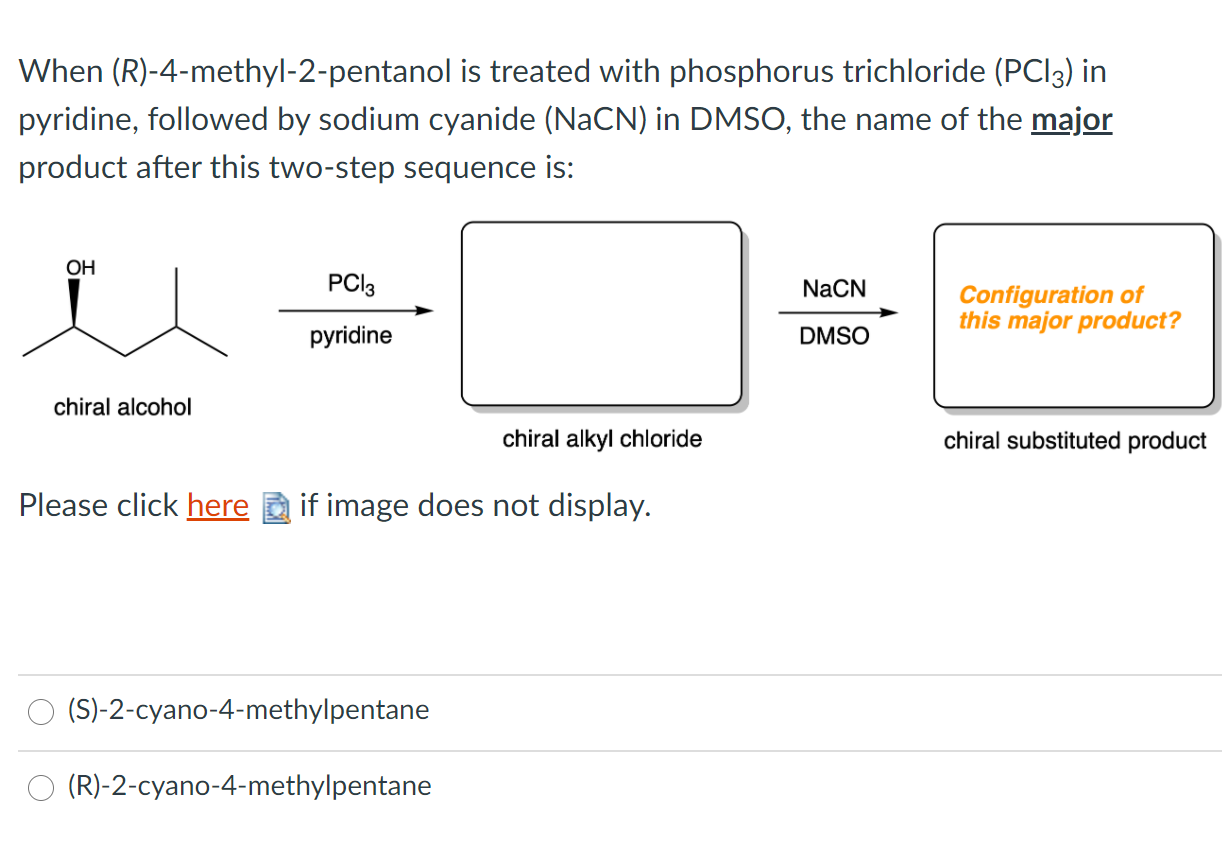



Solved When R 4 Methyl 2 Pentanol Is Treated With Chegg Com




Reaction Of Alcohols With Pcl3 And Pbr3 Class 12 Youtube



1




Complete The Following I R Oh Pcl 5 Rarr Ii R Oh Pcl 3 Rarr



What Is The Mechanism For A Reaction Between Alcohol And Phosphorus Pentachloride Quora




Phosphorus Trichloride An Overview Sciencedirect Topics




Michaelis Arbuzov Reaction Wikipedia




Complete The Following I R Oh Pcl 5 Rarr Ii R Oh Pcl 3 Rarr
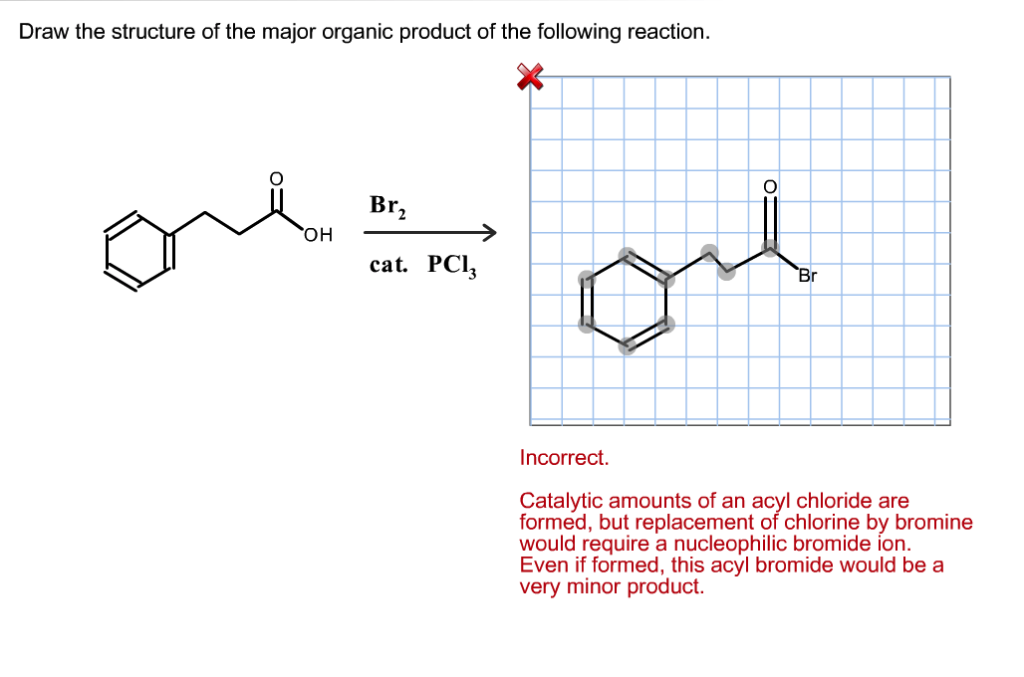



Solved Draw The Structure Of The Major Organic Product Of Chegg Com
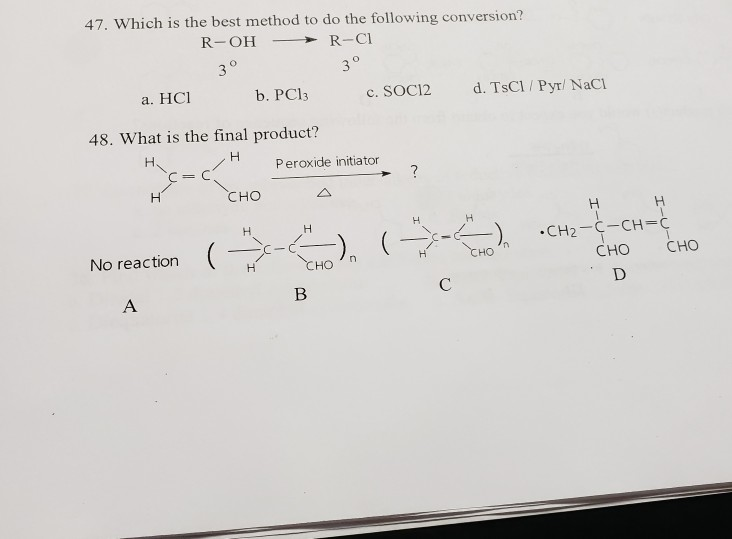



Solved 47 Which Is The Best Method To Do The Following Chegg Com



2




05 Carboxylic Acid Reactions Of Carboxylic Acid With Pcl5 Pcl3 Socl2 Roh P2o5 Nh3 Dry Distilation Youtube




13 Pcl5 Acirc Dagger Oelig Pcl3 Cl2 Agrave Reg Agrave Reg Copy Agrave Macr Agrave
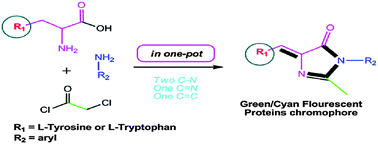



Pcl3 Mediated Synthesis Of Green Cyan Fluorescent Protein Chromophores Using Amino Acids New Journal Of Chemistry Rsc Publishing



Alcohols R O H Classification Ch3 1o 2o 3o Nomenclature Ppt Download



Carboxylic Acids




Write The Chemical Reactions Of C2h5oh With Pcl3 And Pcl5 Separately



Carboxylic Acids R Cooh R Co2h Ppt Download



Solved 13 Given Pcls G Pcl3 G Cl2 G K 0 040 At 450 C Chegg Com
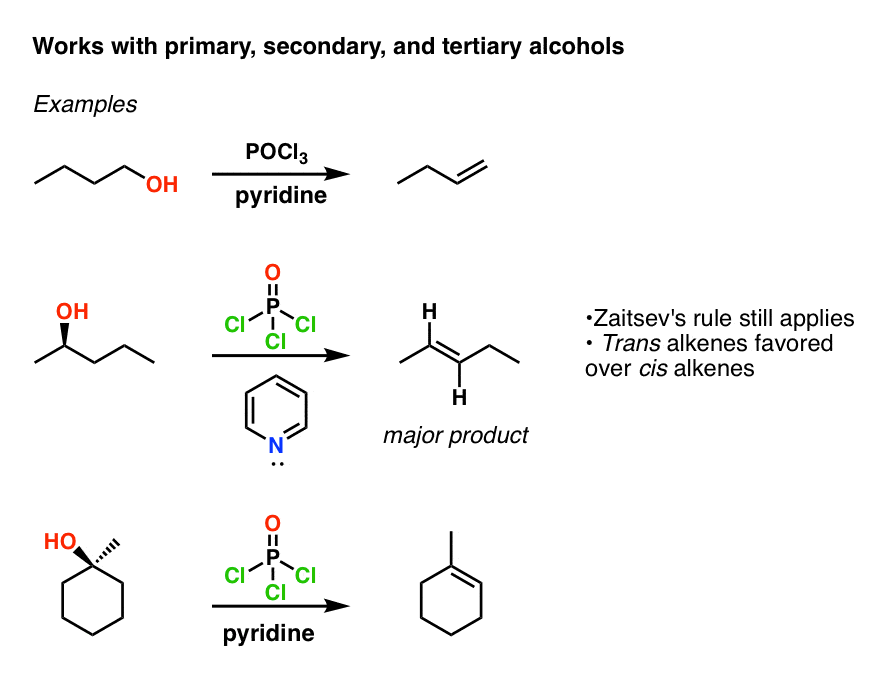



Elimination Of Alcohols To Alkenes With Pocl3 And Pyridine



Solvent Modulated Reactivity Of Pcl 3 With Amines Green Chemistry Rsc Publishing Doi 10 1039 149h




In The Following Reaction Identify The Major Product Oh H Ch Nal Acetone H Ch



What Is The Mechanism For A Reaction Between Alcohol And Phosphorus Pentachloride Quora




Phosphorus Trichloride Wikipedia




Reaction Of Alcohols With Pcl3 And Pbr3 Class 12 Youtube




Pdf First Principles Computational Study On Hydrolysis Of Hazardous Chemicals Phosphorus Trichloride And Oxychloride Pcl 3 And Pocl 3 Catalyzed By Molecular Water Clusters
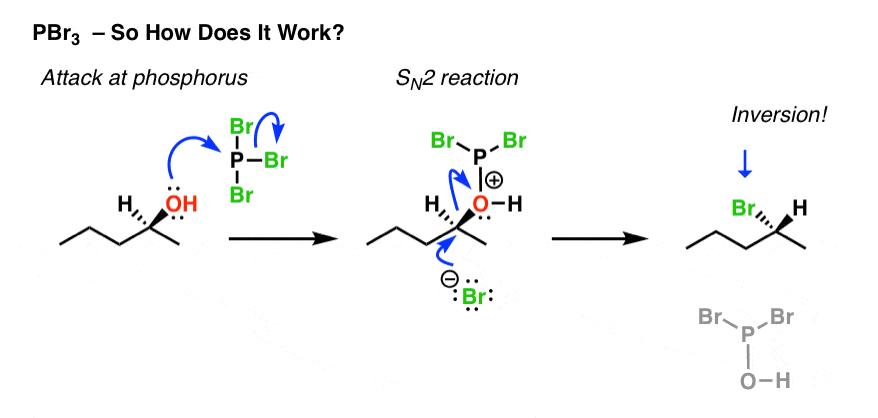



Pbr3 And Socl2 Master Organic Chemistry
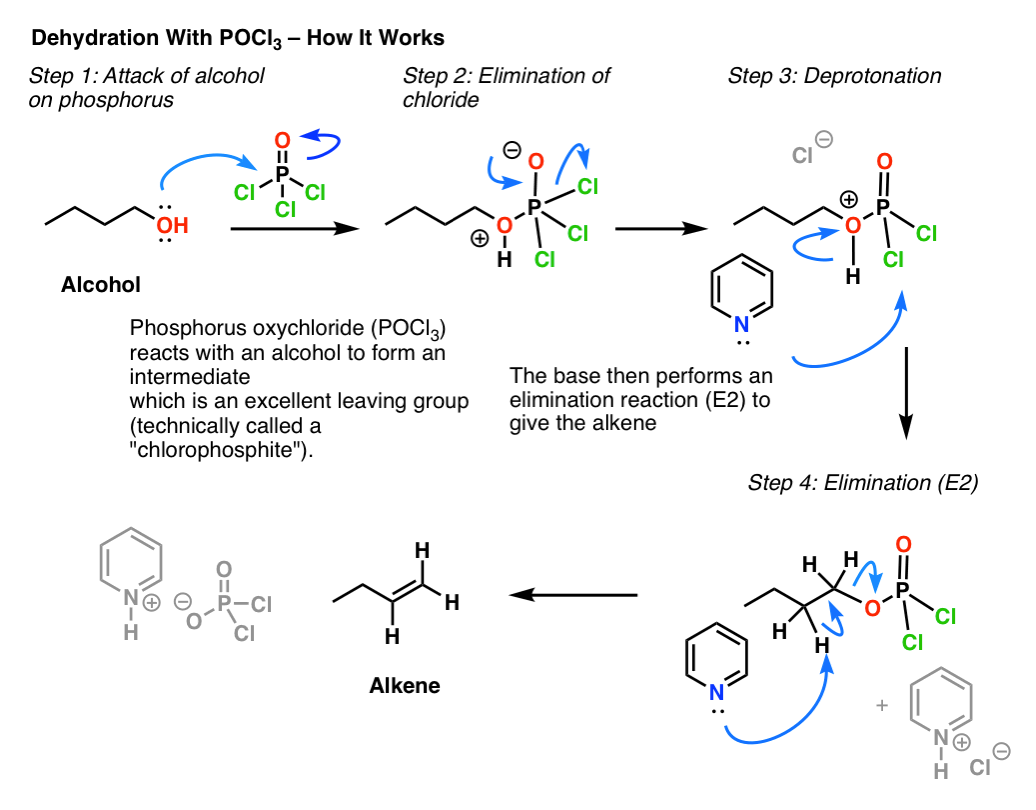



Elimination Of Alcohols To Alkenes With Pocl3 And Pyridine




Reaction Of Carboxylic Acid With Phosphorus Trichloride Chemistry Stack Exchange




Organic Chemistry Preparations From Pcl3 5 Best Method Of Thionyl Chloride Socl2 Facebook



Pbr3 And Socl2 Master Organic Chemistry



More About The Families In Group Ii Ppt Download



Biomolecules Free Full Text Synthesis And Inhibitory Studies Of Phosphonic Acid Analogues Of Homophenylalanine And Phenylalanine Towards Alanyl Aminopeptidases Html



First Synthesis Of Etidronate Partial Amides Starting From Pcl3 Organic Biomolecular Chemistry Rsc Publishing




Investigation Of Reaction Mechanism Of Amino Acids And Phosphorus Trichloride By 31p Nmr And Esi Ms Ms Cao 11 Chinese Journal Of Chemistry Wiley Online Library




Reaction Of Alcohol With Pcl3 Phosphorus Trichloride By Dr Manu Kaushal Youtube
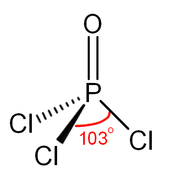



Phosphoryl Chloride Wikipedia



What Is The Mechanism For A Reaction Between Alcohol And Phosphorus Pentachloride Quora




Phosphorus Trichloride An Overview Sciencedirect Topics
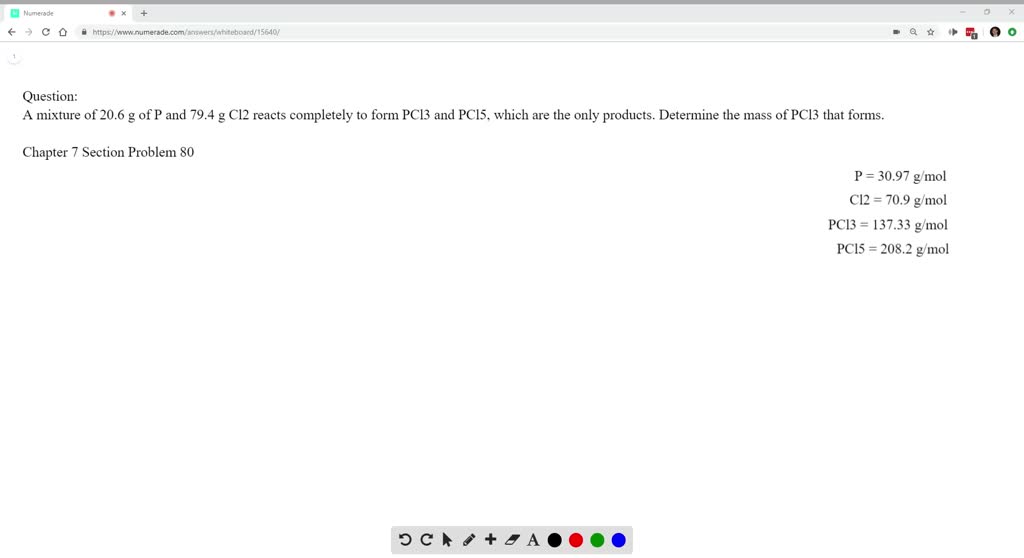



Solved A Mixture Of 6 G Of P And 79 4 G Cl2 Reacts Completely To Form Pcl3 And Pcl5 Which Are The Only Products Determine The Mass Of Pcl3 That Forms




Pdf First Principles Computational Study On Hydrolysis Of Hazardous Chemicals Phosphorus Trichloride And Oxychloride Pcl 3 And Pocl 3 Catalyzed By Molecular Water Clusters
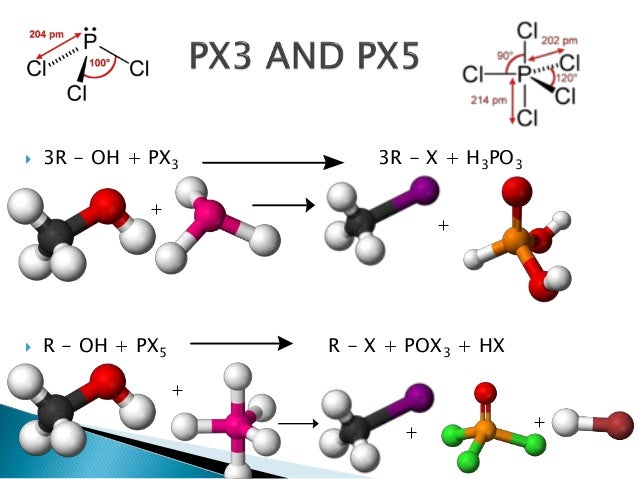



Conversion Of Alcohols To Halide




Organophosphorus Chemistry Without Pcl3 A Bridge From Hypophosphorous Acid To H Phosphonate Diesters Fisher 13 European Journal Of Organic Chemistry Wiley Online Library
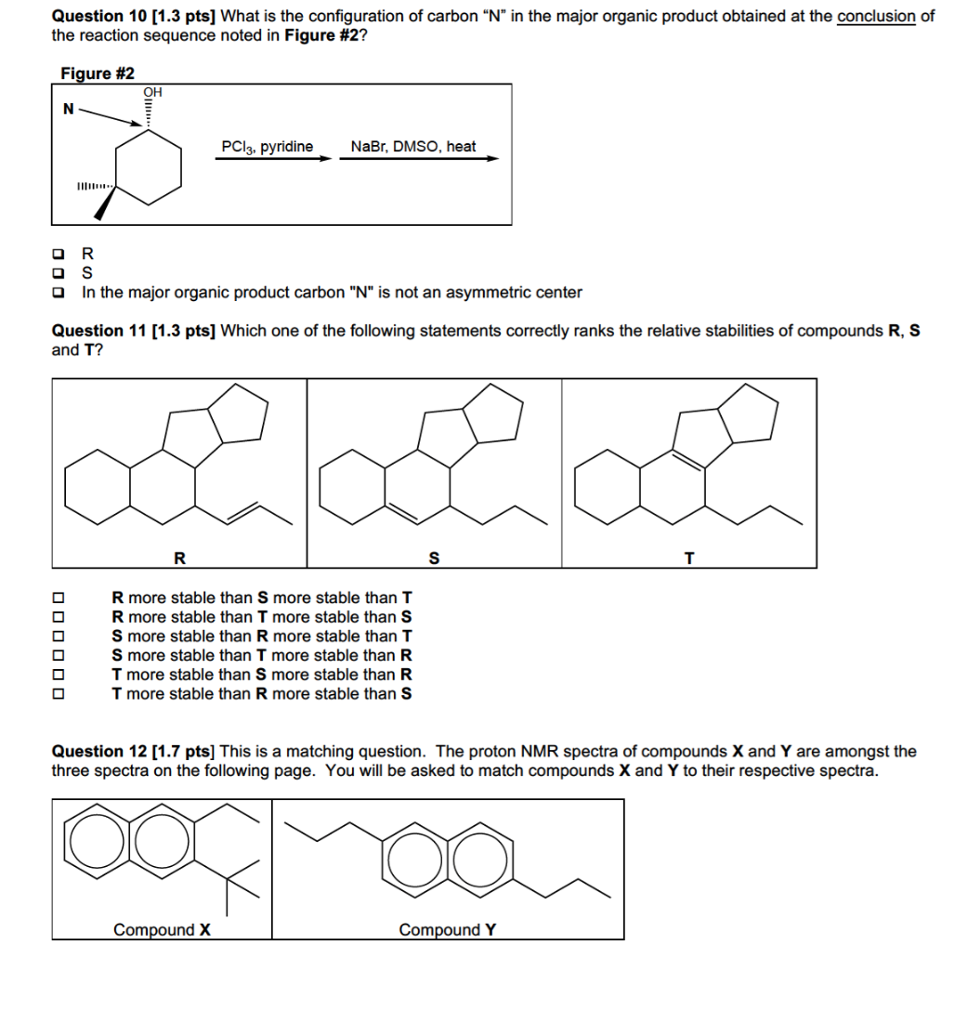



Solved Question 10 1 3 Pts What Is The Configuration Of Chegg Com




Alcohol Reactions Hbr Pbr3 Socl2 Youtube




Pocl3 For Dehydration Of Alcohols Chemistry Steps
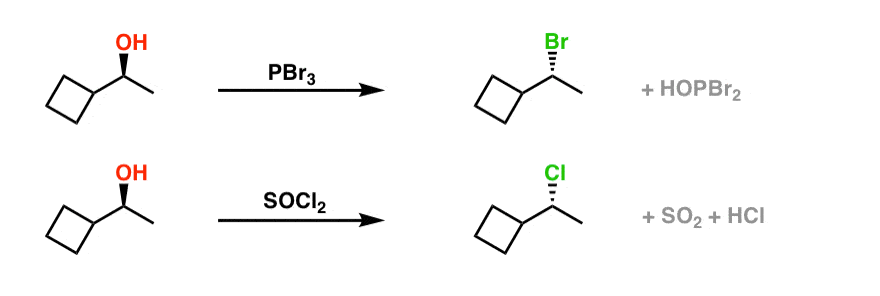



Pbr3 And Socl2 Master Organic Chemistry
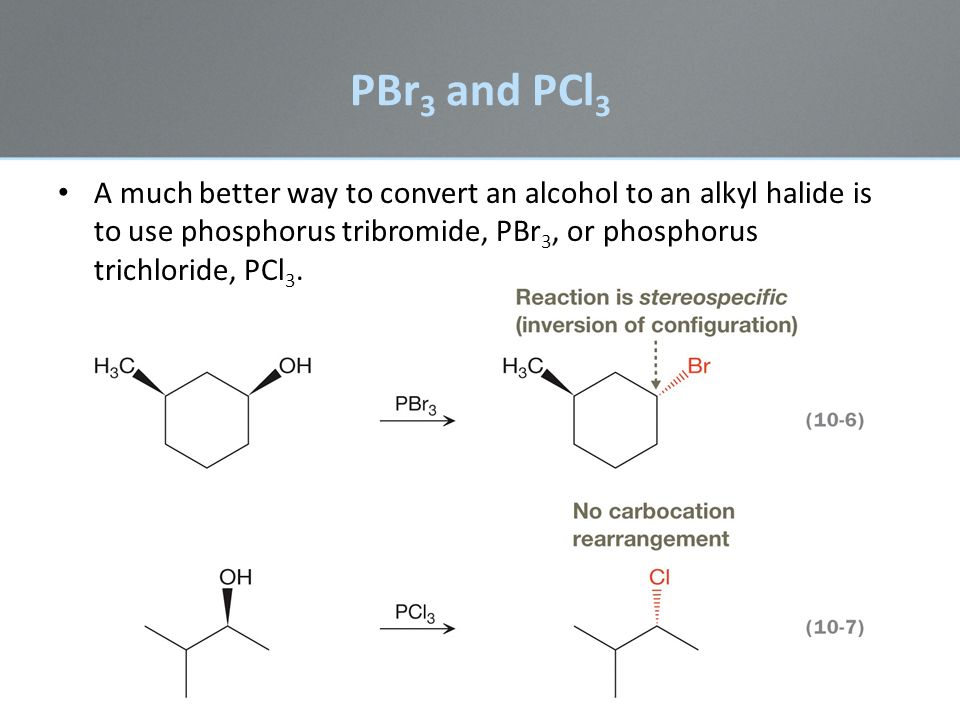



Chapter 10 Lecture Powerpoint Ppt Download
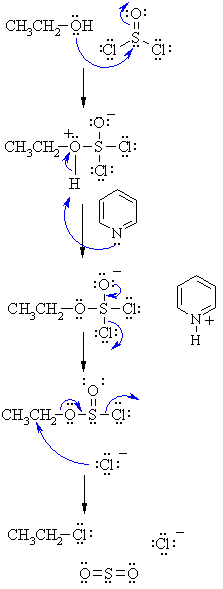



Ch 8 Roh Socl2 Or Px3
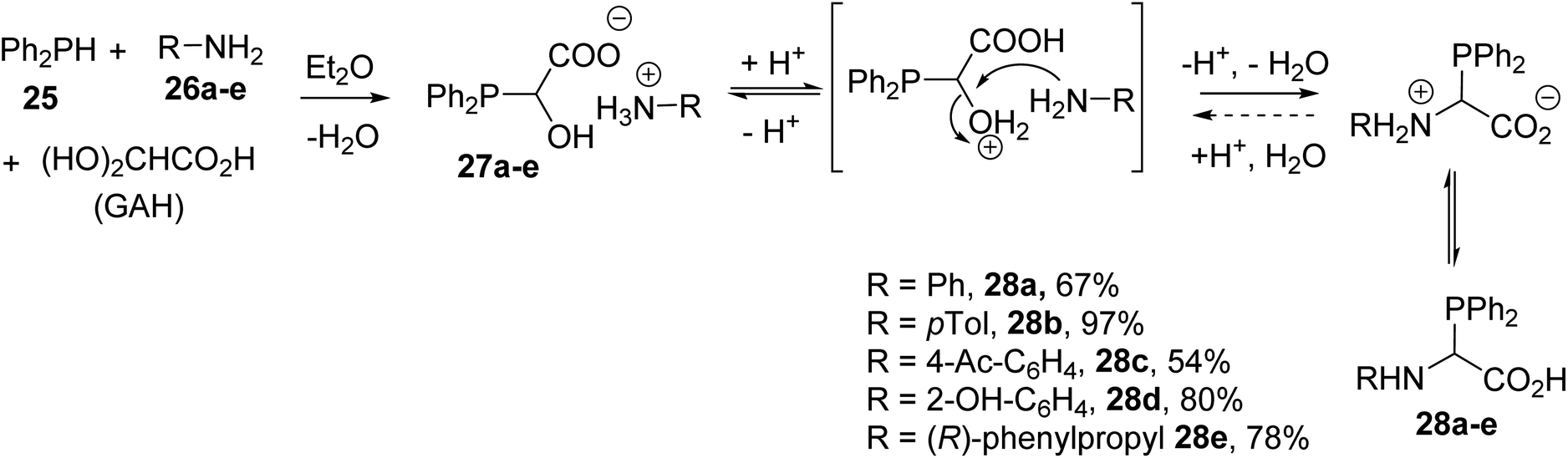



Phosphorus Containing Amino Acids With A P C Bond In The Side Chain Or A P O P S Or P N Bond From Synthesis To Applications Rsc Advances Rsc Publishing Doi 10 1039 C9raj
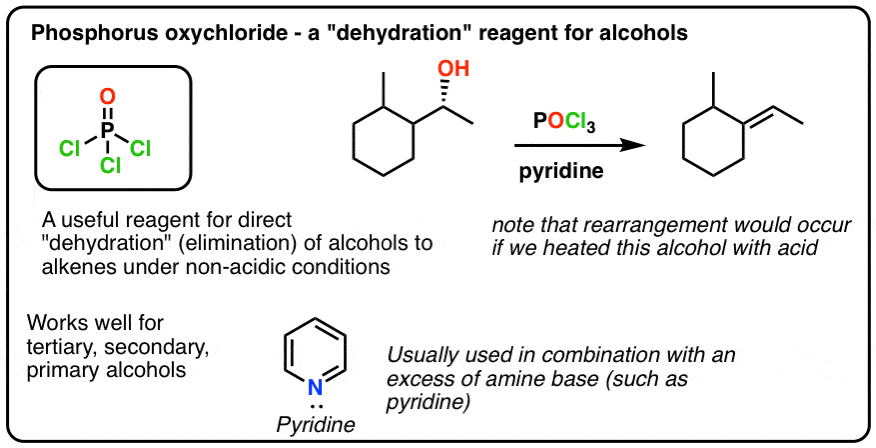



Elimination Of Alcohols To Alkenes With Pocl3 And Pyridine



2




Challenges And Solutions In Phosphinate Chemistry




Synthesis Of The Cyclosal D4tmp Triesters 2 Method A Pcl3 Pyridine Download Scientific Diagram




Phosphorus Trichloride An Overview Sciencedirect Topics



Unit 2 Chemistry Of Carbonyl Compounds Topic Alcohol




Pdf First Principles Computational Study On Hydrolysis Of Hazardous Chemicals Phosphorus Trichloride And Oxychloride Pcl 3 And Pocl 3 Catalyzed By Molecular Water Clusters



What Will Be The Product When Pcl5 Reacts With Ethyl Alcohol Socratic




Reaction Of Alcohols With Pcl5 And Pcl3 Chemistry Stack Exchange



As Conversion Flashcards




Socl2 Reaction With Carboxylic Acids Chemistry Steps




Orgo I Final Review Flashcards Quizlet




What Is The Mechanism Of Reaction Between Alcohol And Pcl5 Chemistry Stack Exchange




Pocl3 For Dehydration Of Alcohols Chemistry Steps



2




13 Pcl5 Acirc Dagger Oelig Pcl3 Cl2 Agrave Reg Agrave Reg Copy Agrave Macr Agrave




Gas Phase Reaction Of Phosphorus Trichloride And Methanol Matrix Isolation Infrared And Dft Studies Sciencedirect
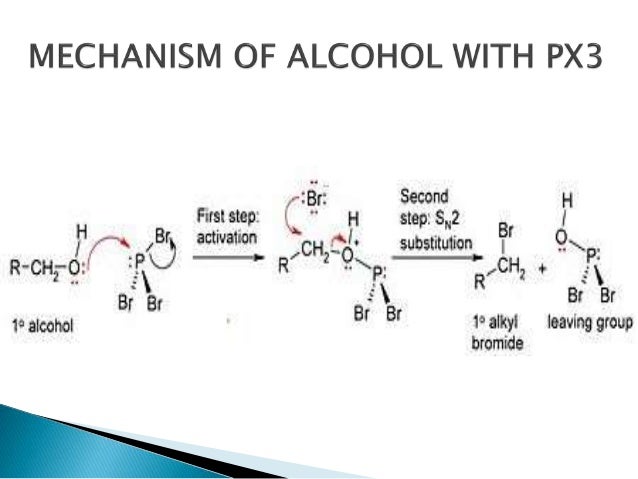



Conversion Of Alcohols To Halide




How To Balance Pcl3 H2o Hcl H3po3 Phosphorous Trichloride Water Youtube




Phosphorus Trichloride An Overview Sciencedirect Topics




Complete The Following I R Oh Pcl 5 Rarr Ii R Oh Pcl 3 Rarr




Pdf First Principles Computational Study On Hydrolysis Of Hazardous Chemicals Phosphorus Trichloride And Oxychloride Pcl 3 And Pocl 3 Catalyzed By Molecular Water Clusters




Pdf First Principles Computational Study On Hydrolysis Of Hazardous Chemicals Phosphorus Trichloride And Oxychloride Pcl 3 And Pocl 3 Catalyzed By Molecular Water Clusters



1




Complete The Following I R Oh Pcl 5 Rarr Ii R Oh Pcl 3 Rarr


